Stefan Hell
| Stefan Hell | |
 Stefan W. Hell | |
| Född | 23 december 1962 Sântana i Arad i Rumänien |
|---|---|
| Nationalitet | Tysk |
| Institutioner | European Molecular Biology Laboratory Max Planck Institute for Biophysical Chemistry German Cancer Research Center |
| Alma mater | Heidelbergs universitet |
| ORCID | 0000-0002-9638-5077 |
| Känd för | Nanoskopi, fluorescensmikroskopi |
| Nämnvärda priser | Gottfried Wilhelm Leibniz Prize (2008) Kavli Prize (2014) Nobelpriset i kemi (2014) |
Stefan Walter Hell, född 23 december 1962 i Sântana i Arad i Rumänien, är en tysk fysiker.[1] 2014 tilldelades han Nobelpriset i kemi[2] tillsammans med Eric Betzig och William Moerner "för utveckling av superupplöst fluorescensmikroskopi".[3]
Hell blev filosofie doktor 1990 vid Heidelbergs universitet. Han är forskningsledare vid Max Planck-Institut für biophysikalische Chemie i Göttingen och chef för Deutsches Krebsforschungszentrum i Heidelberg.
Priser och utmärkelser i urval
- Prize of the International Commission for Optics, 2000
- Berthold Leibinger Innovationspreis, 2002
- Carl-Zeiss Research Award, 2002
- Karl-Heinz-Beckurts-award, 2002
- C. Benz u. G. Daimler-Award of Berlin-Brandenburgisch academy, 2004
- Innovation Award of the German Federal President, 2006
- Julius Springer Prize for Applied Physics 2007
- Gottfried Wilhelm Leibniz Prize, 2008
- Otto-Hahn-Preis, 2009
- Ernst-Hellmut-Vits-Prize, 2010
- Hansen Family Award, 2011
- Körber European Science Prize, 2011[4]
- Meyenburg Prize, 2011[5]
- Science Prize of the Fritz Behrens Foundation 2012
- Paul Karrer Gold Medal, University of Zürich, 2013
- Carus Medal of the Leopoldina, 2013
- Kavli Prize, 2014[6]
- Nobelpriset i kemi, 2014
Källor
- ^ Max Planck Institute for Biophysical Chemistry
- ^ ”The Nobel Prize in Chemistry 2014 - Surpassing the limitations of the light microscope”. Nobelprize.org. Nobel Media AB. 8 oktober 2014. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2014/press.html. Läst 11 oktober 2014.
- ^ Kungliga Vetenskapsakademien (8 oktober 2014). ”Nobelpriset i kemi 2014”. Pressmeddelande. Läst 11 oktober 2014.
- ^ ”Stefan Hell – Körber-Preisträger 2011”. Arkiverad från originalet den 13 oktober 2014. https://web.archive.org/web/20141013021432/http://www.koerber-stiftung.de/wissenschaft/koerber-preis-fuer-die-europaeische-wissenschaft/aktueller-preistraeger.html. Läst 12 oktober 2014.
- ^ ”From microscopy to nanoscopy: 2011 Meyenburg Award goes to Stefan Hell - Pressrelease”. German Cancer Research Center. http://www.dkfz.de/en/presse/pressemitteilungen/2011/dkfz-11-61-Meyenburg-Award-goes-to-Stefan-Hell.php. Läst 12 oktober 2014.
- ^ ”Körber-Preisträger Stefan W. Hell erhält Kavli-Preis”. Körber Stiftung. 3 juni 2014. Arkiverad från originalet den 16 oktober 2014. https://web.archive.org/web/20141016081645/http://www.koerber-stiftung.de/wissenschaft/schwerpunkt-lust-auf-mint/news-details-sp-lust-auf-mint/artikel/koerber-preistraeger-stefan-w-hell-erhaelt-kavli-preis.html. Läst 12 oktober 2014.
| |||||||||||||||||||
|
Media som används på denna webbplats
Författare/Upphovsman: User:Gusme (it:Utente:Gusme), Licens: CC BY-SA 3.0
Vector image of the Nobel prize medal
Författare/Upphovsman: Andy Nestl, Licens: CC BY-SA 3.0
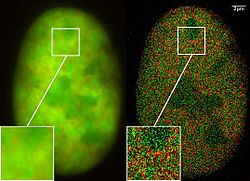
GFP superresolution, optical nanoscopy ( Christoph Cremer, emeritus at Heidelberg university [1])
View of a nucleus of a bone cancer cell: using normal high resolution fluorescence microscopy, it is not possible to distinguish details of its structure (image on the left). Using the two Color Localization Microscopy 2CLM (image on the right) it is possible to localize 70,000 histone molecules (red: RFP-H2A) and 50,000 chromatin remodeling proteins (green: GPF-Snf2H) in a field of view of 470 µm2 with an optical depth of 600 nm. Common fluorescence markers were used.
2CLM is the only optical nanoscopy method that allows position based co-localization of single molecules at high density in a wide field of view using conventional fluorescent proteins such as GFP, YFP, RFP, or other conventional fluorochromes.
Due to its high optical single molecule resolution, 2CLM allows significantly more precise analyses of potential protein interactions than FRET-(Fluorescence Resonance Energy Transfer) technology, which is at present the preferred method for such investigations. This is of particular significance in studies of biomolecular machines (BMMs) within cells: Single BMMS can be analysed, including the number of molecules of a given type; distances between proteins in these BMMs often are substantially greater than those that can be analyzed by FRET (restricted to a maximum distance of only a few nm).
Possible to use conventional, well established and inexpensive fluorescent dyes, from the GFP group, and its dye variants, to the well-known Alexa and fluorescein dyes. Fundamental to SPDMphymod are blinking phenomena (flashes of fluorescence), induced by reversible bleaches (metastable dark states). Individual molecules of the same spectral emission color can be detected.
Publikation: Manuel Gunkel, Fabian Erdel, Karsten Rippe, Paul Lemmer, Rainer Kaufmann, Christoph Hörmann, Roman Amberger and Christoph Cremer: Dual color localization microscopy of cellular nanostructures. In: Biotechnology Journal, 2009, 4, 927-938. ISSN 1860-6768Författare/Upphovsman: Bernd Schuller, Max-Planck-Institut für biophysikalische Chemie, Licens: CC BY-SA 3.0
Portrait of Stefan W. Hell




